A Pharma Company that's driven not just by Research, but by Empathy.
We don’t just make medicine. We make a difference.
Engineering
Our state-of-the-art engineering facilities feature an advanced water purification system that ensures a continuous supply for injection..
Production
The production areas are meticulously cleaned before the start of each batch and after its completion to ensure the highest levels..
Quality policy
Our Quality control team ensures the API received is not only tested but also meets our stringent standards. Similar is the process for testing the finished product..
Dive Deeper »Innovation begins with intention
We strive to lead the global pharmaceutical industry with intent - delivering innovative, affordable, and high-quality medicines. Through purposeful research, integrity, and accessibility, we aim to transform patient care and promote wellness across the world.


The launch of SustaGlar®, our biosimilar long-acting Insulin Glargine, marks a new chapter in advancing global healthcare with quality formulations.
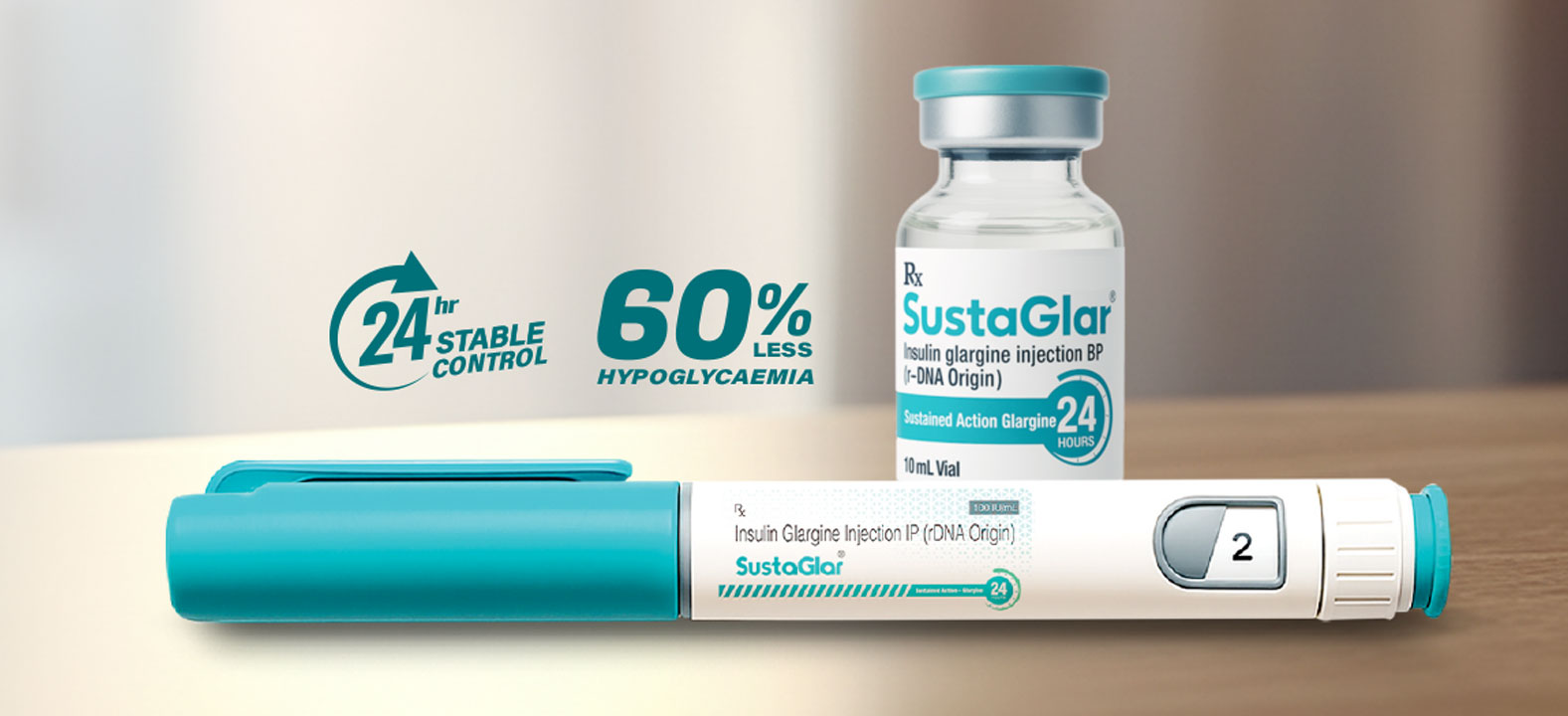

The launch of SustaGlar®, our biosimilar long-acting Insulin Glargine, marks a new chapter in advancing global healthcare with quality formulations.
Global Reach, Dedicated to Your Wellness.
As a company dedicated to health and wellness, we operate with substantial infrastructure, including multiple manufacturing units, supported by a large team of dedicated employees. We are structured into several key divisions to ensure operational excellence.
02
Manufacturing Units

Reginex has manufacturing capabilities that adhere to strict quality norms of WHO and GMP while its intensely researched biosimilars manufacturing unit for insulin glargine mono antibodies is located at Tambaram MEPZ. The Sustaglar manufacturing facility, located in the Special Economic Zone (SEZ) has a 200-litre production capacity, with plans to scale up to 1,000 litres.
1200+
Our Employee

Behind every achievement at Regenix stands a team of 1000+ people who believe in one purpose, i.e. to heal, to innovate, and to make a difference. They are the heart of everything we do. Their passion, expertise, and commitment drive our mission to make quality healthcare accessible and meaningful for all.
05
Our Divisions

Reginex Group now has five successful business verticals including pharma, formulations, manufacturing, marketing and exports. Its Super Med and Regenix Super labs give retail edge with its robust network, while Mur and Mur services import and marketing of chromogenics.
06
Export Countries
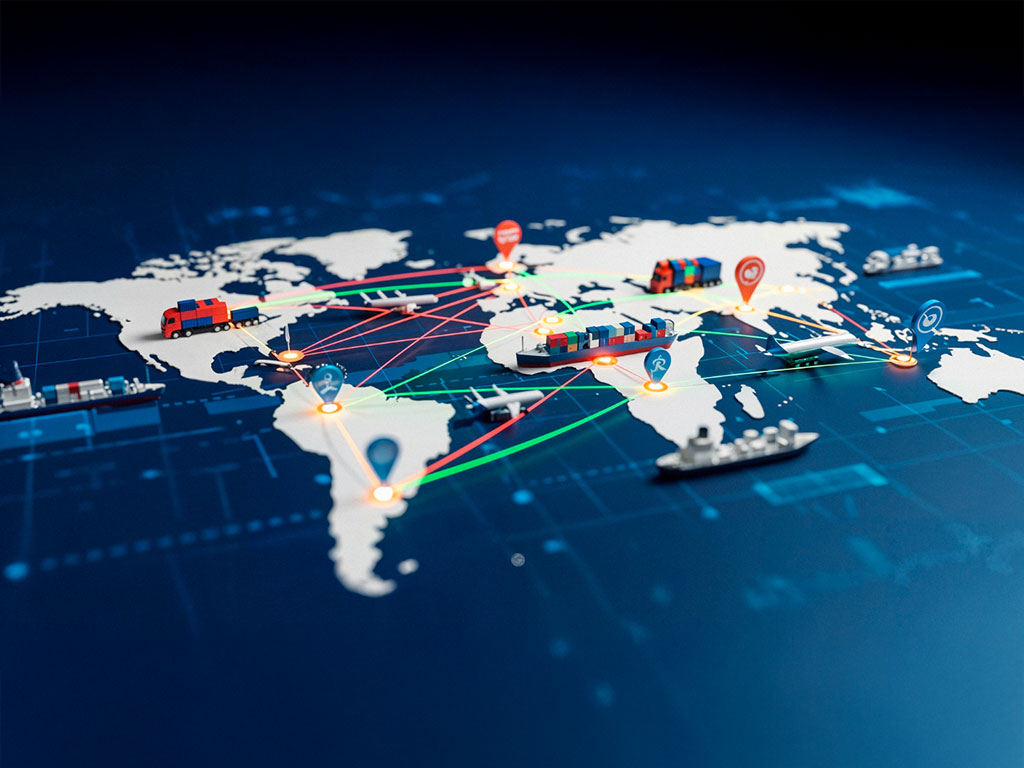
The formulations manufactured at our OSD plant is being exported to South America, Philippines & Nigeria. Several other products are under registration in various other countries. RDL has developed more than 30 CTD & ACTD Dossiers.
Our Journey Through the Years




The Regenix Group today operates across five successful business verticals - pharma, formulations, manufacturing, marketing, and exports, reflecting its strength and diversity within the healthcare ecosystem.



